
Hematology Case Study: A 20 Year Old with Anemia
Case History
A 20 year old Black male with a known history of HbS trait went to the primary care office for a pre-surgical evaluation for elective laparoscopic cholecystectomy for symptomatic cholelithiasis. All physical exam findings were negative. The patient had blood work completed and was found to have mild anemia with microcytosis. On previous imaging, the spleen was noted to be slightly enlarged. Further workup included a peripheral blood smear, finding target cells, microspherocytes, folded cells, and rod-shaped Hb C crystals (see image below). No sickled RBCs were noted.
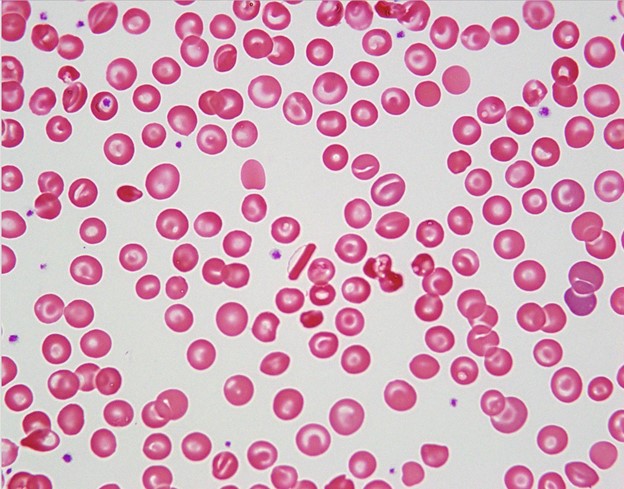
Discussion
Hemoglobin C disease is an intrinsic red cell disorder caused by Hemoglobin C (Hb C). Hb C is a variant of normal Hemoglobin A (Hb A) that results from a missense mutation in the β-globin protein, replacing the glutamic acid at position 6 with a lysine molecule. The disease can be either in the homozygous state (Hb CC) or in the heterozygous states (Hb AC or Hb SC). The origin of this mutation was traced back to West Africa and is found to confer protection against severe manifestations of malaria. In the United States, the Hb C allele is prevalent in about 1-2% of the African American population. There is an equal incidence between gender, and the incidence of the homozygous disease (i.e., Hb CC) is only 0.02%. Nevertheless, these statistics may be under-representative, since the disease is generally asymptomatic.
Heterozygous individuals with Hb AC usually show no symptoms, while homozygous individuals with Hb CC can have mild hemolytic anemia, jaundice, and splenomegaly. When Hb C is combined with other hemoglobinopathies, such as Hemoglobin S (Hb S), more serious complications can result. Hb S is similar to HbC in that it arises from a missense mutation; ie, a valine is substituted for the glutamic acid at the 6th position on the β-globin protein. As a result of this mutation, HbS abnormally polymerizes when in the presence of low oxygen tension, leaving the red blood cells (RBCs) rigid and irregularly shaped. Sickle cell disease (SCD) typically is a result of homozygous Hb S mutations (i.e., Hb SS), but the disease can also come from Hb SC.
All clinical features of Hb SS can be seen in Hb SC, including painful vaso-occlusive crises, chronic hemolytic anemia, stroke, acute chest syndrome, etc. Nevertheless, Hb SC is generally a milder disease. The complications from HbSC disease are less severe and less frequent when compared to Hb SS. Fortunately, unlike those with Hb SS disease, patients with Hb SC disease do not experience autosplenectomy, but they can develop splenomegaly. There are two complications that occur in HbSC disease occur more frequently than in HbSS disease, and they include proliferative sickle cell retinopathy and avascular necrosis of the femoral head (the latter case presents especially in peripartum women). Therefore, patients with HbSC disease should follow up with ophthalmology and obstetrics to monitor these complications. Furthermore, patients with Hb SC disease can vary in the severity of symptoms and the resulting complications. For example, some patients may develop a severe anemia and require blood transfusions; whereas, other patients are minimally affected by the disease. Overall, patients with Hb SC disease tend to have a better life expectancy compared to those with Hb SS disease. Patients with Hb SS disease have an average life expectancy of 40 years, while those with Hb SC disease are expected to live into their 60s and 70s. In contrast to Hb SS and Hb SC disease, Hb CC disease does not have an increase in mortality. As mentioned earlier, Hb CC disease results only in mild anemia, asymptomatic splenomegaly, and largely absent clinical symptoms.
Pathologic features of Hb SC and Hb CC diseases can be seen on a peripheral blood smear (PBS). Hb CC disease does not show sickled RBCs, while Hb SC can show sickled RBCs though very rarely. More importantly, Hb C is prone to polymerize into characteristic crystals. Depending on the zygosity of the individual, the crystals take on a defining shape. In heterozygous individuals (Hb SC), the crystals are found as irregular, amorphous, or bent appearing, and the RBCs can take on a “spiked and hooked” appearance. In homozygous individuals (Hb CC), the crystals are elongate, straight, and uniformly dense (as seen in the case above). In addition to crystals, the PBS shows numerous target cells, scattered folded cells, and microspherocytes.
Ancillary studies for diagnosis of these diseases include Hb variant analysis, such as electrophoresis and high-pressure liquid chromatography. Cellulose acetate (alkaline) electrophoresis is a standard method used to separate Hb A, Hb A2, Hb F, Hb C, Hb S, and other variants according to charge. Some hemoglobin variants comigrate using this described method, so citrate agar (acid) electrophoresis can be used additionally to distinguish between these variants. In Hb CC disease, analysis shows nearly all Hb C with small amounts of Hb F (i.e., fetal hemoglobin) and HbA2 (i.e., a normal variant of Hb A, in which the hemoglobin molecule is made up of 2 α chains and 2 δ chains). In Hb SC disease, analysis demonstrates almost equal amounts of Hb S and Hb C.
References
- Aster JC, Pozdnyakova O, Kutok JL. Hematopathology: A Volume in the High Yield Pathology Series. Philadelphia, PA: Saunders, an imprint of Elsevier Inc.; 2013.
- Gao J, Monaghan SA. Hematopathology. Chapter 1: Red Blood Cell/Hemoglobin Disorders. 3rd edition. Philadelphia, PA: Elsevier; 2018.
- Karna B, Jha SK, Al Zaabi E. Hemoglobin C Disease. 2020 Jun 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 32644469.
- Mitton BA. Hemoglobin C Disease. Medscape, 9 Nov. 2019, emedicine.medscape.com/article/200853-overview.
- Saunthararajah Y, Vichinsky EP. Hematology: Basic Principles and Practice. Chapter 42: Sickle Cell Disease: Clinical Features and Management. Philadelphia, PA: Elsevier; 2018.
